Background:The incidence of Delayed Hemolytic Transfusion Reaction (DHTR) is 0.04% but is higher in patients with sickle cell disease (SCD). Although it has been reported in hematopoietic progenitor cell (HPC) transplantation, its incidence in SCD following nonmyeloablative HPC transplantation remains unknown. We report a recipient with blood group O who received a nonmyeloablative HPC transplantation with blood group A three years ago. She is now typing as blood group A and developed a DHTR after receiving group A red blood cells (RBC).
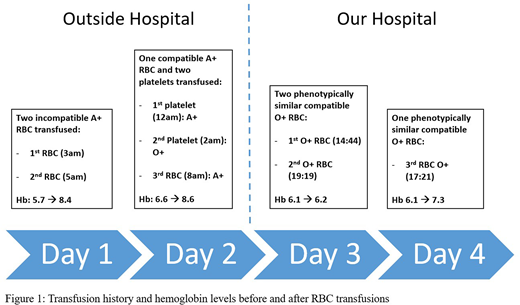
Case Report:A 42-year-old female was transferred from an outside hospital (OSH) for management of Acute T-Cell Lymphoblastic Leukemia. Three years prior, she received a group A+ matched sibling nonmyeloablative HPC transplantation for SCD. Her original blood group was O+, and she had a history of clinically significant RBC alloantibodies (anti-E, anti-C, anti-Goa, anti-Kell, and anti-Jkb). On admission to OSH (Day 1), the type and screen showed group A+ without ABO discrepancy. The antibody screen was negative. The OSH blood bank was unaware of her immunohematologic history, because it was not communicated to them by the OSH hematologists, and the only documented diagnosis was that of acute leukemia. Per SOP, OSH performed only immediate spin crossmatch. She was transfused three units of A+ RBC at OSH in preparation for her transfer to us on Day 3 (Fig. 1). At admission to our hospital, her laboratory parameters were suggestive of both tumor lysis syndrome and hemolysis. Her type and screen specimen was grossly hemolyzed. She typed as Group A+. The Direct Antiglobulin Test (DAT) was positive with polyspecific antisera, positive for IgG and negative for bound complement factors. Antibody screen was negative except for the anti-Goa. In the eluate, we identified anti-A or anti-A,B, which were not differentiated for lack of clinical implications. Per our request, OSH retrospectively performed a pre-transfusion DAT, which was negative, and an AHG crossmatch of the pre-transfusion sample. The results showed that the RBC transfused at OSH on Day 1 were incompatible (1+) and those transfused on Day 2 after O+ platelet transfusion were compatible (Fig. 1). This confirmed that the eluted antibodies were not passively transferred from the platelet transfusion but were rather isoagglutinins from the patient's own plasma of her original blood type O. Chimerism studies indicted the presence of only 25% donor CD3 and 30% donor myeloid cells. Further studies at our institution confirmed the hemolysis to be due to anti-A/A,B and not anti-Goa. Antibody titers of the patient's plasma with A1 and A2 cells were low (1) and negative, respectively. The titer of the eluate with A1 cells and B cells was 4 and 2, respectively. The crossmatch of the patient's plasma with A1 cells was negative at immediate spin and 37oC but positive at the IgG phase, which explains the negative crossmatch at immediate spin at OSH. We believe that the exposure of the 2 incompatible A+ RBC at OSH prompted an anamnestic response, causing the hemolysis of the transfused RBC. Subsequently, the patient required the transfusion of 3 additional RBC. Due to the presence of positive DAT developed after 24 hours of transfusion (on Day 3), the positive elution test, inadequate rise of post-transfusion Hb level and rapid fall in Hb back to the concentration pre-transfusion (Fig. 1), this reaction is best classified as a definitive DHTR in accordance with the CDC hemovigilance guidelines.
Conclusion:This case is a warning for the perfect storm from the combination of HPC transplantation and SCD. Our patient had a history of transplantation for SCD and clinically significant alloantibodies. OSH blood bank was not aware of her immunohematologic history, and she received incompatible RBC units that were crossmatched at immediate spin only. She subsequently developed a DHTR which was clinically significant, requiring blood transfusion. This is a good reminder of the importance of good communication between clinicians and the transfusion services. The need for caution when using electronic crossmatch or immediate spin is also important, especially in this era of transplantation for SCD. The absence of RBC antibodies cannot be assumed when a transfusion history is lacking. Increasing awareness, prevention and early recognition and treatment are essential to avoid the potential fatal complication of hemolytic transfusion reactions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal